The following information on tetanus in the horse is provided for veterinarians:
Tetanus is caused by exotoxins produced by the bacterium Clostridium tetani, a motile anerobic gram-positive bacillus.
Clostridium tetani is a ubiquitous soil inhabitant and can be isolated from the faeces of many normal horses.
Spores are produced which are highly resistant to heat and are not destroyed by boiling.
Autoclaving at 115 degrees Celsius for 20 minutes will destroy the spores.
The most common route of infection is inoculation of wounds with Clostridium tetani spores from the environment.
Puncture wounds contaminated with soil or manure are likely to cause tetanus.
Other potential sources of Clostridium tetani infection:
(i) Contaminated surgical sites such as castration wounds;
(ii) Postpartum uterus, particularly following foaling trauma, retained aferbirth or uterine infections;
(iii) Hoof abscess;
(iv) Injection site abscesses; and,
(v) Neonatal foal umbilical cords.
However, studies have reported that up to 50% of horses with tetanus have no obvious causative wounds.
Horses appear particularly susceptible to developing clinical signs of tetanus compared to many other animal speceis.
Tetanus remains an important disease worldwide for the equine population.
Due to its devastating clinical effects in horses research efforts over the last 100 years have focused on developing effective short-term and long-term preventatives.
Animal species susceptibility to tetanus toxin varies but the horse is considered one of the most sensitive of all to the toxin.
Two prinicpal toxins called tetanolysin and tetanospasmin are produced by Clostridium tetani.
Tetanolysin damages viable tissue and promotes favourable anerobic conditions for bacterial growth.
Tetanospasmin targets neuromuscular junctions throughout the body and is responsible for the clinical signs of tetanus. Its action results in sustained excitatory discharge of motor neurons causing muscle rigidity and muscle spasms.
Tetanospasmin has been shown to travel along axons at 75 to 250 mm/day until it enters the central nervous system leading to autonomic dysfunction.
Neuronal binding is considered irreversible and recovery requires growth of new nerve terminals that may take weeks to months.
The time from wound incoluation to development of first clinical signs may take 1 to 60 days, but is usually 7 to 21 days. The average incubation time was found to be 9 days in a series of 18 horses.
Generalised signs of tetanus are usually seen in the horse.
The first signs of tetanus are associated with rigidity of the muscles around the head and neck. Increased masticatory muscle tone, rigidity of facial expression and neck stiffness are typical. Prolapse of the third eyelid begins at this stage. The head and neck are held in an extended position, and the face has a fixed anxious expression. The ears and commissures of the lips may be retracted caudally and the upper eyelids elevated. The mouth may be clamped shut and jaws cannot be prised open (lockjaw).
In mild cases the signs may be restricted to the head and neck. Signs of dysphagia may be present because of pharyngeal involvement and rigidity extends to the muscles of the lumbs, trunk and tail.
Horses may adopt a 'sawhorse' posture with rigid extension of the neck, back and limbs with elevation of the tailhead.
In all cases of generalised tetanus tonic spasms develop within days of the onset of muscle rigidity. These can be initiated by loud noises, touching the horse or by the horses own voluntary movements. Horses may appear hyperesponsive and hyperaesthetic. Spasms may prevent the horse from reaching to the ground for food or water.
Muscle spasms can be disabling enough to cast an adult horse into lateral recumbency and once down it is difficult for a horse to regain its feet.
Localised tetanus has rarely been described in horses.
Sweating and tachycardia comonly occur in horses presenting with generalized tetanus.
Reduced intestinal activity, infrequent defecation, colic and dehydration are frequent complications.
Death usually occurs because of euthanisa of recumbent horses on welfare grounds with uncontrollable muscle contractions and associated musculoskeletal injuries.
The diagnosis of tetanus in the horse is based upon clinical signs in the context of poor, absent or unknown toxoid vaccination or tetanus antitoxin treatment.
Gram stains of infected wounds can reveal the gram-positive bacilli but are seldom attempted in horses.

Therapy for tetanus in horses should have the objectives of:
(i) Provision of a safe and quiet environment;
(ii) Neutralisation of residual toxin;
(iii) Eliminate causative bacteria;
(iv) Muscle relaxtion;
(v) Relief of pain; and
(vi) General supportive care.
Neutralisation of any circulating toxin is essential and this is achieved by administration of tetanus antitoxin (TAT), although bound toxin is likely to be inaccessible to TAT.
TAT can be given i.m., i.v., or s.c.
TAT doses of 10,000 IU daily for 3 to 5 days have been recommended.
Intrathecal administration of TAT has been attempted in a small number of horses but the results are inconclusive.
Antibiotics such as penicillin and metronidazole have been used to successfully control wound infection in horses with tetanus.
Muscle relaxation may be achieved with various sedatives (diazepam, xylazine, midazolam, etc).
Parenteral administration of magnesium has been shown in humans to reduce the dose of sedation required. Monitoring of serum Mg concentration is recommended.
Intravenous or stomach-tubed fluids may be required to maintain hydration during critical stages.
The fatality rate reported for horses is at least 50% and up to 75% in some studies.
For horses showing signs of tetanus, the prognosis for survival is poor if:
(i) The horse develops a rapid onset of severe clinical signs; or,
(ii) The horse becomes recumbent within 24-48 hours.
For horses showing signs of tetanus, the prognosis for survival is good if:
(i) The initial wound was treated promptly with antibiotics and TAT early in the course; or,
(ii) The disease has stabilised and not progressed after 4-5 days; or,
(iii) The horse remains ambulatory and able to eat and drink; or,
(iv) The horse received a tetanus toxoid vaccination in the year before disease.
In general studies of large numbers of cases have reported that horses initially presenting recumbent with rapid and severe clinical signs have a poor prognosis for recovery.
Non-vaccinated horses that recover from clinical tetanus are unlikley to develop any significant protective immunity due to the low concentration of tetanus toxin in circulation and should receive tetanus toxoid to ensure long term protection.
Tetanus is highly preventable disease through the strategic use of tetanus antitoxin (TAT) and toxoid vaccination.
Vaccination can provide reliable long term protection. It is important to observe the specific manufacturers recommendations for the primary immunisation course and that regular booster doses are given otherwise immunity may be inadequate.
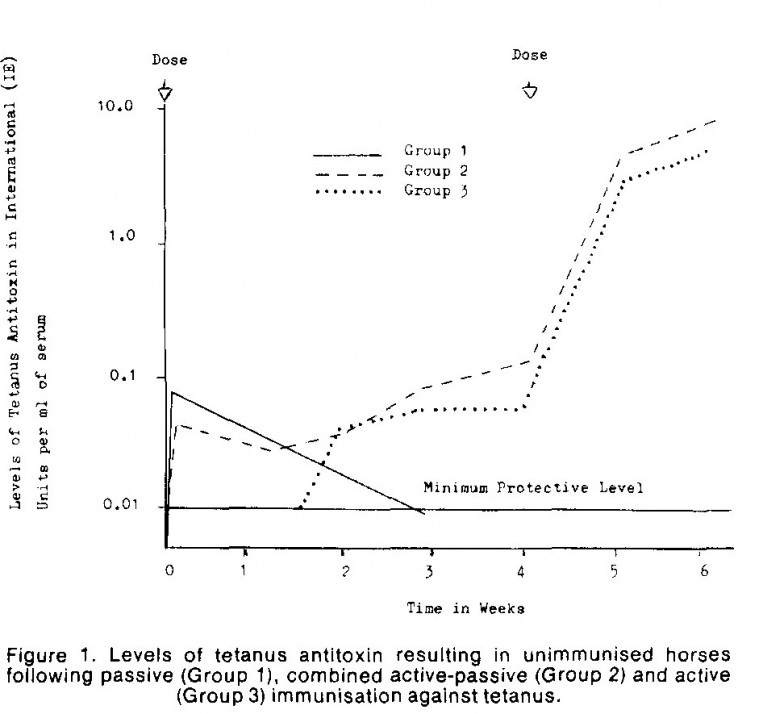
It is advisable to administer TAT and toxoid vaccination concurrently (see graph below), with separate syringes at separate sites. Research undertakin in Australia (Liefman et al 1980) demonstrated that there was no interference with toxoid immunisation when tetanus antitoxin has given at the same time but on different sides of the neck.
Vaccination of late pregnant mares with tetanus toxoid will boost the mare's immunity and ensure passive transfer of tetanus protection via colostrum to the foal.
Injection of TAT to a non-vaccinated 'at-risk' horse provides short term immunity for 2-3 weeks.
TAT is indicated in any wounded horse that has not been vaccinated in the previous year.
TAT is indicated in foals born from non-vaccinated mares.
TAT is indicated in neonatal foals with failure of passive transfer of antibodies via colostrum.
TAT may be indicated in foals at birth on properties with known high risk of tetanus.
Maternal Antibody Inteference with Tetanus Toxoid Vaccination
Immunisation of late pregnant mares will boost their immunity and ensure adequate colostral transfer of antibodies to tetanus toxin. However, maternal antibodies against tetanus have been shown to persist in foals for many months after birth and can inhibit the response to tetanus toxoid vaccination. Vaccination of foals under 3 months of age, born to vaccinated mares, has been shown to result in inadequate and short lived immunity. This has led to the more recent recommendation that tetanus toxoid vaccination should begin no earlier than 6 months of age. Foals born to non-immune mares could begin a primary vaccination course at a younger age, such as 1-4 months. A three dose primary series in foals is the most effective strategy with 4 weeks interval between first and second doses and 3 to 5 months interval for the third dose.
Horses immunised as yearlings appear to develop better immune responses to toxoid vaccine, likely because maternal antibody has decline and no longer inhibits responses. In this context, a two dose course of toxoid vaccine appears adqeuate to generate sufficient and lasting immunity.